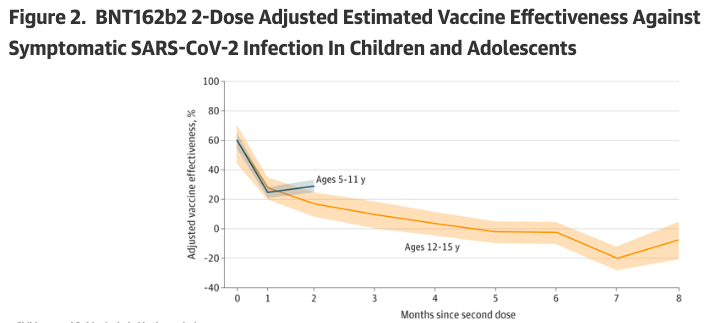
“Among children and adolescents, estimated VE [vaccine effectiveness] for 2 doses of BNT162b2 against symptomatic infection was modest and decreased rapidly,”
Twitter has removed a warning that it had added to a study by the Centers for Disease Control and Prevention (CDC) as part of its campaign to limit what it claims to be “potentially harmful and misleading content.”
Twitter and other social networking giants are known to have worked closely with the CDC in controlling the narrative during the pandemic, including suppression of alleged misinformation, in emails obtained by Judicial Watch through a Freedom of Information Act (FOIA) in July 2021.
In one of the emails, Twitter offered the CDC free advertising on its Promoted Trend and Promoted Spotlight Trend, with an estimated value of $75,000 and $150,000 respectively.
While in a different email dated March 26, 2020, a CDC representative asked the social media network for assistance in getting its partner organization verified status so that “the COVID-19 messaging they are posted gets the same resonance as other verified accounts.”
Phil Kerpen, president of American Commitment, posted about the recent blunder on Twitter, saying that the network had “slapped a warning on the **Journal of the American Medical Association** for the wrongthink crime of publishing data showing rapid waning of COVID vaccines in children.” Kerpen had posted the study back in May to his account.
The Journal of the American Medical Association (JAMA) is a peer-reviewed medical journal that published the CDC study on May 13, 2022.
When clicking on the link provided on Kerpen’s post on June 8, a message appeared, stating “Warning: this link may be unsafe.”
The CDC study examined the effectiveness of the two-dose Pfizer COVID-19 injection in children (ages 5 to 11) and adolescents (ages 12 to 15) between December 2021 and February 2022 during the Omicron variant predominance.
The authors found that the estimated effectiveness of the Pfizer shot declined significantly in the two groups by the second month after dose two: 29 percent in children and 17 percent in adolescents. When the mRNA shots were issued emergency use authorization prior to any predominant variant, the effectiveness of two doses was 100 percent in children and 91 percent in adolescents.
“Among children and adolescents, estimated VE [vaccine effectiveness] for 2 doses of BNT162b2 against symptomatic infection was modest and decreased rapidly,” the authors wrote.
In the graph provided in the study, near the fifth month after dose two, the vaccine’s effectiveness was negative in the adolescent group, meaning the vaccinated were more likely to have symptomatic COVID-19 than the unvaccinated.


